Updated: February 15, 2022


Urinary Catheters
Urinary catheters are hollow tubing that insert up the urethra to the bladder for waste removal. The tubing is firm but highly flexible for negotiating the urethra pathway. These bladder devices provide relief for people who are unable to empty their bladder normally. Bladders that do not empty regularly allow urine to build up and cause the patient pain and may damage the kidneys. The need for catheter use is often temporary, but sometimes an injury or severe urological illness can lead to an extended period and sometimes permanently. These medical devices come in many sizes and versions to best meet the needs of individual urology patients. This article examines the construction of modern urinary catheters, their use, and the advantages that Cure catheters offer. A side-by-side comparison chart displays some of the top selling catheters on the market.
Indications
- Prolonged patient immobilization
- Lack of urination control
- Urinary incontinence
- Acute urinary retention
- Bladder outlet obstruction
- To aid in healing open perineal or sacral wounds
- Prevent skin break-down
- Urologic surgical procedures
- Prolonged surgical procedures
- Surgeries with large-volume infusions or diuretics1
Contraindications
- Urethra trauma
- Scrotal hematoma
- Pelvic fracture
- High riding prostate
- High suspicion of a urethral tear
- Blood at the meatus of the urethra2
Inappropriate Uses
- Substitute for nursing care of a patient with incontinence
- For obtaining samples for diagnostic evaluation when the patient can urinate voluntarily3
Catheter Construction
Urinary catheter construction is primarily of three different materials--polyvinyl chloride (PVC), silicone, and latex. Silicone and latex are the most widely used uretic catheters. Many catheters come with a coating to provide easier insertion and to prevent infections. These coatings include hydrophilic, silicone-elastomer, polytetrafluoroethylene, silver, and PTFE-based Teflon. The coatings reduce friction and reduce the instance of inflammation and illnesses.
PVC
PVC is polyvinyl chloride plastic used in many medical products. Medical tubing, exam gloves, urine leg bags, blood collection bags, and many other items use PVC. It is a transparent material that allows observation to view the color of urine. It is pliable and soft to support insertion through the urethra. It is also a firm material that is stiff enough to push to its ultimate destination.
Advantages
- Polished or cold-punched eyelets or drainage holes facilitate less resistance during insertion
- Clear material allows observation
- Latex-free
- Color-coded funnels identify product size
- Funnels facilitate gripping and draining
Disadvantages
- Often made with DEHP or DiNP, known carcinogens that gas-off from plastic
- Damages the environment
- DEHP and DiNP adversely affects the liver, kidneys, lungs, heart, and reproductive tract
Top-Selling PVC Brands
Silicone
Silicone is an inert synthetic polymer that is highly flexible and resistant to external influences. It offers both thermal and chemical stability. Silicone provides biocompatibility, it does not react to substances in your body. This material is also bio-durable and will not break-down in contact with other common chemicals found in the body.
Advantages
- Rigid flexibility for easy insertion
- Resists reactions to body fluids
- Biocompatible
- Less harmful to cells
- Less likely to form deposits
- Resistant to bacterial colonization
- Less chance of infection
- Resistant to chemical reaction
- Latex-free
- Use for up to 90 days
Disadvantages
- A weak polymer that is prone to fractures
- Some instances of catheter tips breaking off in the bladder
- May be toxic for some users
- More expensive
Top Selling Silicone Brands
Latex
This tubing is the most flexible and soft drainage instrument. It is a less expensive option. It is available with many different types of coatings to help reduce friction and adverse reactions. The most popular outer layers include silicone, silicone elastomer, and hydrogel.
Advantages
- Highly flexible material
- Easy to insert
- Less expensive
- Used for up to 28-days
Disadvantages
- Subject to form mineral deposits or encrustation on the outer surface
- Encrusted outer surface causes considerable friction and may rupture tissue upon removal
- Numerous people have latex allergies
- Higher risk of infections
- Higher risk of hypersensitivity reactions
- Prone to irritating the urinary tract
Top Selling Latex Catheters

Latex and Phthalates Danger
Latex Allergies
Latex is a secretion from the hevea brasiliensis tree and many other plants as a protective fluid against insects. This secretion converts to natural rubber through a vulcanization process. There is also synthetic latex made of a polymer in a water-based liquid. Latex products are widely used both in homes and in hospitals. Latex allergy is an increasingly worrying problem for health professionals, not least allergy to latex urinary catheters. Medical studies demonstrate that many individuals may experience adverse reactions to the use of latex products.
Anyone with a history of frequent exposure to latex is at risk. Latex could be considered a habitual allergen. The use of latex urinary catheters should be avoided in patients who are catheterized on a daily basis.4
Natural rubber latex (NRL) is a ubiquitous allergen as it is a component of > 40,000 products in everyday life. Latex allergy might be attributed to skin contact or inhalation of latex particles. Latex allergy is an IgE-mediated hypersensitivity to NRL, presenting a wide range of clinical symptoms such as angioedema, swelling, cough, asthma, and anaphylactic reactions....
An immediate allergic reaction may occur within minutes of coming into contact with latex. Symptoms of a reaction include hives, wheezing, coughing, shortness of breath, sneezing, nasal congestion, runny nose, conjunctivitis, nasal, palatal, or ocular itching, urticaria, naso-rhinitis, asthma, and hypotension.
Hives can appear anywhere on the body and not necessarily at the point where direct contact with the latex occurred. Immediate reactions can be life-threatening when blood pressure drops, airways blocked, and the throat closes. This condition can eventually progress to anaphylaxis.
Avoidance of the provoking agent (latex allergen) is the most effective way to manage any allergy. Latex-free synthetic rubber such as neoprene, nitrile, styrene-butadiene rubber (SBR), butyl, and vitron are polymers that are available as alternatives to natural rubber. There are no naturally occurring proteins in them and they are not responsible for latex allergy.5
Catheter-associated urinary tract infections (CAUTIs) are the most common kind of nosocomial infection. Recent years have seen a significant increase in the numbers of infections caused by yeasts of the genus Candida. The adherence of a microorganism to the host surface is a decisive factor in the success of colonization and the pathogenesis of infection.
The results demonstrated that the latex catheter facilitated adherence more than the silicon catheter. The adherence of the C. albicans was significantly greater than C. parapsilosis on latex, but it was similar on silicon.6
A nursing study observed that latex allergies are an increasingly worrying problem for health professionals, not [the] least urinary catheters. The pathophysiology of latex allergies, the toxicity leading to urethritis, stricture formation, and crustation emergence requires screening patients for latex catheter risk factors.7
The Foley catheter, introduced in the mid-1930s and originally manufactured from latex, is still the most commonly used device for the management of urinary incontinence (UI). Despite the passage of time, there are still problems associated with the use of these devices. It is currently estimated that the management and treatment of UI cost the UK National Health Service (NHS) in the order of £500 million per annum. Faced with the known demographic changes in the adult population these costs will continue to rise for the foreseeable future.8
Phthalate Allergies
Phthalates are the subject of increasing concern owing to their effects on human health. Proof of their toxicity has led European regulators to restrict the use of certain phthalates in numerous products. MDs containing phthalates classified as class 1 or 2 carcinogenic and mutagenic chemical substances and/or toxic for reproduction [DEHP, di-butyl-phthalate (DBP), benzyl-butyl-phthalate (BBP)] must be labeled as such. For at-risk populations (children, pregnant or breastfeeding women), the use of these substances must be justified. In France, since July 2015, the use of MDs with DEHP is banned in pediatric, neonatal, and maternity departments.9
Di(2‐ethylhexyl)phthalate (DEHP) is a reproductive and developmental toxicant in animals and a suspected endocrine modulator in humans. There is widespread exposure to DEHP in the general population. Patients can be additionally exposed through DEHP‐containing medical devices.
DEHP is used as a plasticizer for polyvinyl chloride (PVC). Soft PVC can consist of up to 40% DEHP. Thus, DEHP is a major component, e.g. in flooring, carpeting, roofing, vinyl wall covering, upholstery, wire and cable sheathing, clothing, packaging, and toys. In the non‐PVC‐field DEHP is used in detergents, industrial solvents, wetting agents, or lubricating oils, e.g. for lacquers, colors, or adhesives.
As these plasticizers are not chemically bound to the polymer, they leach, migrate, or gas out into the atmosphere, into foodstuff or directly into body fluids, thus exposing the environment and people.... [These] oxidized metabolites have rather long half‐lives of elimination (10–24 h) compared with the simple monoester MEHP. These long half‐lives of elimination indicate that under chronic exposure conditions for the ubiquitously present phthalates, DEHP or some of its oxidized metabolites might accumulate in the body. Furthermore, recent studies give first hints that these oxidized metabolites might be the ultimate developmental DEHP toxicants.
Biomonitoring data show that the general population and children are ubiquitously exposed to DEHP. Based on urinary DEHP metabolite excretion, children are significantly more exposed than adults. Calculation models show that international preventive limit values like the TDI and RfD can be exceeded on an individual basis. For children, we assumed that similar to adults, almost the total oral DEHP dose is absorbed. If children had lower absorption rates for DEHP compared with adults, their (already higher) urinary metabolite excretion only led to an underestimation but not to an overestimation of the actual DEHP dose.10
DEHP leaches in varying concentrations into solutions stored in PVC medical devices. Certain populations, including dialysis patients and hemophiliacs may have long‐term exposures to clinically important doses of DEHP, while others, such as neonates and the developing fetus, may have exposures at critical points in development. In vivo and in vitro research links DEHP or its metabolites to a range of adverse effects in the liver, reproductive tract, kidneys, lungs, and heart.11,12
Di-Ethylhexyl Phthalate (DEHP)
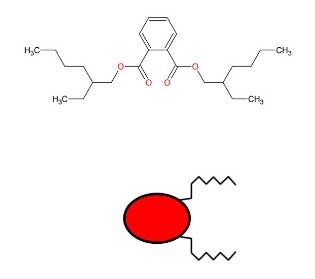
DEHP is a plasticizer used in the manufacturing of plastic. It softens plastics like Polyvinyl Chloride (PVC) to make medical devices more flexible. Studies with animals demonstrated damage to the liver, kidneys, and reproductive organs. DEPH is a carcinogen, according to the Centers for Disease Control.
Polyvinyl chloride (PVC) plasticizers are widely present in the environment. These compounds are mainly used to soften PVC and to improve the flexibility of a broad range of objects including food packaging, toys, flooring, shower curtains, and cables. In particular, they enter into the composition of medical devices (MD) made with PVC such as tubing and medical gloves
Until 2010, the most commonly used plasticizer in MDs was di-(2-ethylhexyl) phthalate (DEHP), which can account for 30 to 40% of the weight of plastics for medical use. As they have no chemical bond with plastic materials, phthalates are easily released into the environment and DEHP can be released from MDs
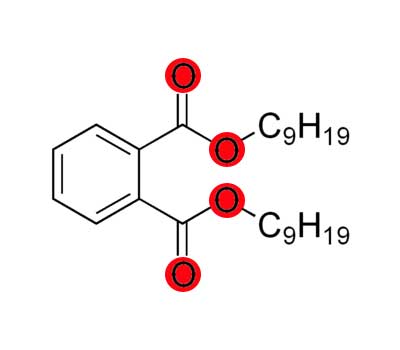
Diisononyl Phthalate (DiNP)
DiNP is a phthalate in plastic catalyst to make items flexible. It is one of the most widely used plasticizers in manufacturing. Several animal studies have demonstrated that DiNP exposure increased the incidence of liver, spleen, and kidney tumors. DiNP induces antiandrogenic effects in animals and, therefore, can contribute to the cumulative risk from exposure to other antiandrogenic phthalates.13
In 2008, the US passed a new law, the Consumer Products Safety Improvement Act, which banned the use of six phthalates in children’s toys: DiNP, DEHP, DBP, BzBP, DiDP, and DnOP.14,15
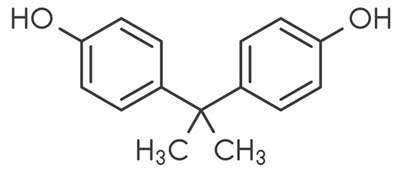
Bisphenol A (BPA)
Bisphenol A (BPA) is used to manufacture polycarbonate plastics. Testing for BPA shows widespread exposure to the U.S. population. There is extensive evidence that bisphenol A (BPA) is related to a wide range of adverse health effects based on both human and experimental animal studies.16
BPA is an additive for the elimination of surfeit of hydrochloric acid during the polyvinyl chloride (PVC) production.... BPA is metabolized in the liver to form bisphenol A glucuronide and mostly in this form is excreted with urine. Due to its phenolic structure BPA has been shown to interact with estrogen receptors and to act as agonist or antagonist via estrogen receptor (ER) dependent signalling pathways. Therefore, BPA has been shown to play a role in the pathogenesis of several endocrine disorders including female and male infertility, precocious puberty, hormone dependent tumours such as breast and prostate cancer and several metabolic disorders including polycystic ovary syndrome (PCOS). Because of the constant, daily exposure and its tendency [towards] bio-accumulation this substance is harmful to catheter patients.17, 18,,19
There is an inhibitory effect of BPA on nuclear estrogen (E2) production in granulosa cells of developing follicles that disrupt normal development to the antral follicles via suppression of E2 in granulosa cells of developing follicles during the menstrual cycle followed by a reduction in the number of oocytes retrieved in in-vitro fertilization (IVF) patients. Several studies corroborate an inverse association between serum and/or urinary BPA concentration and the IVF outcome.20
Catheter Coatings
Coatings
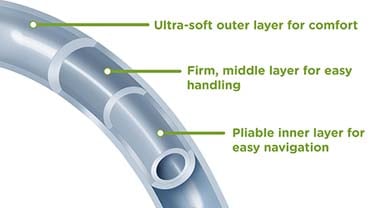
Urinary catheters are commonly used in chronic care facilities and senior facilities throughout the world. Many of the problems associated with catheters include infection, encrustation, tissue trauma, and inflammation. These issues can be correlated to the catheter surface. Many of the shortcomings with catheter surfaces can be alleviated by simply adding a friction-reducing coating. Several coatings are now in use, including hydrogel, Teflon,® and silicone. Hydrophilic coatings appear to be the best option. Hydrogel provides a smooth, soft surface with lubricating properties.21
Hydrophilic
A hydrophilic surface coating is a polymer layer that readily absorbs liquid. When immersed in water, it swells to a smooth, slick film. The film provides for easier insertion into the body. A hydrogel-coated latex urinary catheter has a smooth surface with fissures, ripples, and furrows filled with a slick cover. The topography of the hydrophilic coated latex surface compares with a 100% silicone catheter.22
Silicone
A silicone coating provides a smooth surface. A silicone outer layer on a latex catheter provides a smooth covering to help reduce patient discomfort and irritation during insertion.
Silicone-Elastomer
A silicone-elastomer coating is a hydrophobic material that repels moisture. This layer helps to protect the urethral from irritation. It offers a glassy surface for a less painful insertion.
Polytetrafluoroethylene
Polytetrafluoroethylene (PTFE) or Teflon is a fluorocarbon-based polymer that lines the catheter to reduce friction by providing a smooth surface. PTFE coatings increase torque control, flexion, and stiffness to provides easier insertion.
Antimicrobial Agents
Silver is an antibacterial agent. Adding silver or another antimicrobial such as chlorhexidine, Triclosan, enzymes, nitric oxide, or liposomes to the coating reduces the risk of infection and inflammation. Antimicrobial coatings help protect from urinary tract infections (UTI) that often plaque long-term catheterization patients.
More than 75% of hospital-acquired or nosocomial urinary tract infections began by urinary catheters. These devices treated 15 to 25% of hospitalized patients. Among other purposes, urinary catheters are primarily used for draining urine after surgeries and for urinary incontinence. During catheter-associated urinary tract infections, bacteria travel up to the bladder and cause infection.23

The Cure Medical Catheter Difference
Having discussed what catheterization is, the indications for use, contraindications, construction, phthalates, and the various coating options, we will now turn to the role of Cure catheters and how they compare with the other brand offerings. Cure catheters offer significant advantages over other branded products. These advantages include PVC construction but without the use of DEHP, DINP, BPA, or natural rubber latex. Cure catheters do not kink when bent. They come with smooth, polished eyelets that make them easy to insert and extract. Packaged ready-to-use, the packing material is minimal for a lower environmental impact. Below is a summary of the Cure benefits and features.
Advantages of Cure Catheters
- ✓ Smooth, polished eyelets
- ✓ Packaged ready-to-use
- ✓ Straight or coude options
- ✓ Male and female options
- ✓ Sizes 8 to 18 French
- ✓ Pre-lubricated (Ultra, Hydrophilic) options
- ✓ CoverAll application
- ✓ No roll connector funnel facilitates draining
- ✓ Will not kink when bent
- ✓ DEHP-free
- ✓ DINP-free
- ✓ BPA-free
- ✓ Natural Rubber Latex-free
- ✓ Small, flexible packaging
- ✓ Minimal disposal material
Comparing the Top Catheter Brands
| Intermittent | Ultra | Closed System |
SpeediCath Compact |
VarPro Plus |
Apogee | GentleCath | Magic3 | Red Rubber Robinson |
|
| Manufacturer | Cure | Cure | Cure | Coloplast | Holister | Holister | ConvaTec | Bard | Teleflex |
| Construction | PVC | PVC | PVC | PVC | PVC | PVC | PVC | Silicone | Latex |
| Coating Options | Hydrophilic | Hydrophilic | Hydrophilic | Hydrophilic | Hydrophilic | Hydrophilic | Hydrophilic | Hydrophilic Antibacterial |
- |
| Eyelets | Smooth & Polished |
Smooth & Polished |
Smooth & Polished |
Smooth & Fire Polished |
Smooth | Smooth & Fire Polished |
Polished | 4-small | 2 |
| Tip Options | Straight Coude |
Straight Coude |
Straight Coude |
Straight Coude |
Straight | Straight Coude |
Straight Tiemann |
Straight | Straight |
| No-Drip | Yes | Yes | Yes | - | - | - | - | - | - |
| CoverAll | Yes | Yes | Yes | - | - | - | - | - | - |
| Easy Gripper Sleeve | Yes | Yes | Yes | - | - | - | - | - | - |
| No Roll Funnel | Yes | Yes | Yes | - | - | - | - | - | - |
| Kink-Free | Yes | Yes | Yes | - | - | - | - | Resistant | - |
| DEHP-Free | Yes | Yes | Yes | Yes | Yes | - | Yes | Yes | - |
| DINP-Free | Yes | Yes | Yes | Yes | Yes | - | - | Yes | - |
| BPA-Free | Yes | Yes | Yes | Yes | Yes | - | - | Yes | - |
| NR Latex-Free | Yes | Yes | Yes | - | Yes | Yes | Yes | Yes | No |
| Other Features | BZK Wipe Swabsticks Gloves Sterile Wipe Underpad |
Pull-Ring | Anti-Reflux Valve |
Introducer Tip |
No-Touch Sleeve |
3-layers | X-ray Opaque |
||
| Introducer Tip |
Adhesive Dot |
Hydration | FeelClean Technology |
||||||
| Integrated Bag |
Integrated Bag |
Integrated Bag |
Product Videos
Dr. Gerard Henry on DEHP Video (1:21 minutes)
Hydrophilic Cure Video (1:29 minutes)
Cure Ultra Video (1:11 minutes)
Cure Ultra Coude Video (1:11 minutes)
Footnotes
- 1 Guideline for Prevention of Catheter-Associated Urinary Tract Infections. Infection Control. Centers for Disease Control and Prevention. (2009) Page 2. (Last Accessed September-9-2020)
- 2 Urinary Catheter Insertion. Urinary Catheter. Department of Emergency Medicine. University of Ottawa. (Last Accessed September-9-2020)
- 3 Guidelines, Page 2. (Last Accessed September-9-2020)
- 4 Moneret-Vautrin, Denise-Anne, et al. Prospective study of risk factors in natural rubber latex hypersensitivity. Journal of allergy and clinical immunology 92.5 (1993): Page 668. (Last Accessed September-9-2020)
- 5 Deval, Ravi, et al. Natural rubber latex allergy. Indian Journal of Dermatology, Venereology, and Leprology 74.4 (2008): 304. (Last Accessed September-9-2020)
- 6 Tamura, N. K., A. Gasparetto, and T. I. E. Svidzinski. Evaluation of the adherence of Candida species to urinary catheters. Mycopathologia 156.4 (2003): 269-272. (Last Accessed September-9-2020)
- 7 Woodward, Sue. Complications of allergies to latex urinary catheters. British Journal of Nursing 6.14 (1997): 786-793. (Last Accessed September-9-2020)
- 8 Lawrence, E. L., and I. G. Turner. Materials for urinary catheters: a review of their history and development in the UK. Medical engineering & physics 27.6 (2005): 443-453. (Last Accessed September-9-2020)
- 9 Marie, Cécile, et al. Exposure of hospitalised pregnant women to plasticizers contained in medical devices. BMC women's health 17.1 (2017): 45. (Last Accessed September-9-2020)
- 10 Koch, Holger M., Ralf Preuss, and J. Di Angerer. Di (2‐ethylhexyl) phthalate (DEHP): human metabolism and internal exposure–an update and latest results 1." International journal of andrology 29.1 (2006): 155-165. (Last Accessed September-9-2020)
- 11 Tickner, Joel A., et al. Health risks posed by use of Di‐2‐ethylhexyl phthalate (DEHP) in PVC medical devices: A critical review. American journal of industrial medicine 39.1 (2001): 100-111. (Last Accessed September-9-2020)
- 12 ToxFAQs for Di(2-ethylhexyl)phthalate (DEHP). Toxic Substances Portal. Centers for Disease Control. (Last Accessed September-9-2020)
- 13 Diisononyl Phthalate (DiNP). Vermont Department of Health. (Last Accessed September-9-2020)
- 14 Wang, Yu, Hongkai Zhu, and Kurunthachalam Kannan. A review of biomonitoring of phthalate exposures. Toxics 7.2 (2019): 21. (Last Accessed September-9-2020)
- 15 CONSUMER PRODUCT SAFETY IMPROVEMENT ACT OF 2008. PUBLIC LAW 110–314—AUG. 14, 2008. Consumer Product Safety Improvement Act of 2008. Commerce and trade. 15 USC 2051. (Last Accessed September-9-2020)
- 16 vom Saal, Frederick S., and Wade V. Welshons. Evidence that bisphenol A (BPA) can be accurately measured without contamination in human serum and urine, and that BPA causes numerous hazards from multiple routes of exposure. Molecular and cellular endocrinology 398.1-2 (2014): 101-113. (Last Accessed September-9-2020)
- 17 Konieczna, Aleksandra, Aleksandra Rutkowska, and D. Rachon. Health risk of exposure to Bisphenol A (BPA). Roczniki Państwowego Zakładu Higieny 66.1 (2015). (Last Accessed September-9-2020)
- 18 Bisphenol A (BPA). National Institute of Environmental Health Sciences. (Last Accessed September-9-2020)
- 19 Bisphenol A (BPA) Factsheet. National Biomonitoring Program. Centers for Disease Control. (Last Accessed September-9-2020)
- 20 Cho, Seung Hee, et al. Urinary bisphenol A versus serum bisphenol A concentration and ovarian reproductive outcomes among IVF patients: Which is a better biomarker of BPA exposure?. Molecular & Cellular Toxicology 13.4 (2017): 351-359. (Last Accessed September-9-2020)
- 21 Graiver, D., R. L. Durall, and T. Okada. Surface morphology and friction coefficient of various types of Foley catheter. Biomaterials 14.6 (1993): 465-469. (Last Accessed September-9-2020)
- 22 Cox, A. J. Effect of a hydrogel coating on the surface topography of latex-based urinary catheters: an SEM study. Biomaterials 8.6 (1987): 500-502. (Last Accessed September-9-2020)
- 23 Singha, Priyadarshini, Jason Locklin, and Hitesh Handa. A review of the recent advances in antimicrobial coatings for urinary catheters. Acta biomaterialia 50 (2017): 20-40. (Last Accessed September-9-2020)
Cure Medical Literature
 Instruction Guide for catheterization for women.
Instruction Guide for catheterization for women. Instructions for women using closed system.
Instructions for women using closed system. Instructions for Men for intermittent catheterization.
Instructions for Men for intermittent catheterization. Hydrophilic Caterization instructions for men.
Hydrophilic Caterization instructions for men. Closed System Insertion instructions for men.
Closed System Insertion instructions for men. Intermittent Closed System Catheters instructions for parents of girls.
Intermittent Closed System Catheters instructions for parents of girls. Use Instructions for Intermittent Closed System Catheters for parents of boys.
Use Instructions for Intermittent Closed System Catheters for parents of boys. Free Cure Catheter Samples see and feel the difference.
Free Cure Catheter Samples see and feel the difference.
Medical Studies
 Silva, Manori J., et al. Urinary oxidative metabolites of di (2-ethylhexyl) phthalate in humans. Toxicology 219.1-3 (2006): 22-32. (Last Accessed September-9-2020)
Silva, Manori J., et al. Urinary oxidative metabolites of di (2-ethylhexyl) phthalate in humans. Toxicology 219.1-3 (2006): 22-32. (Last Accessed September-9-2020) Vesterberg, Anna, Magnus Hedenmark, and A. M. Vass. PVC in medical devices. An Inventory of PVC and Phthalates Containing Devices Used in Health Care (2005). (Last Accessed September-9-2020)
Vesterberg, Anna, Magnus Hedenmark, and A. M. Vass. PVC in medical devices. An Inventory of PVC and Phthalates Containing Devices Used in Health Care (2005). (Last Accessed September-9-2020) Johns, Lauren E., et al. Exposure assessment issues in epidemiology studies of phthalates. Environment international 85 (2015): 27-39. (Last Accessed September-9-2020)
Johns, Lauren E., et al. Exposure assessment issues in epidemiology studies of phthalates. Environment international 85 (2015): 27-39. (Last Accessed September-9-2020) California Proposition 65. State of California Legistature. (1987) (Last Accessed September-9-2020)
California Proposition 65. State of California Legistature. (1987) (Last Accessed September-9-2020) Phthalates Factsheet. National Biomonitoring Program. Centers for Disease Control. (Last Accessed September-9-2020)
Phthalates Factsheet. National Biomonitoring Program. Centers for Disease Control. (Last Accessed September-9-2020) Ikarashi, Yoshiaki, et al. Comparative studies by cell culture and in vivo implantation test on the toxicity of natural rubber latex materials. Journal of biomedical materials research 26.3 (1992): 339-356 (Last Accessed September-9-2020)
Ikarashi, Yoshiaki, et al. Comparative studies by cell culture and in vivo implantation test on the toxicity of natural rubber latex materials. Journal of biomedical materials research 26.3 (1992): 339-356 (Last Accessed September-9-2020) Graham, D. T., et al. In vivo validation of a cell culture test for biocompatibility testing of urinary catheters. Journal of biomedical materials research 18.9 (1984): 1125-1135. (Last Accessed September-9-2020)
Graham, D. T., et al. In vivo validation of a cell culture test for biocompatibility testing of urinary catheters. Journal of biomedical materials research 18.9 (1984): 1125-1135. (Last Accessed September-9-2020) Ha, U-Syn, and Yong-Hyun Cho. Catheter-associated urinary tract infections: new aspects of novel urinary catheters. International journal of antimicrobial agents 28.6 (2006): 485-490. (Last Accessed September-9-2020)
Ha, U-Syn, and Yong-Hyun Cho. Catheter-associated urinary tract infections: new aspects of novel urinary catheters. International journal of antimicrobial agents 28.6 (2006): 485-490. (Last Accessed September-9-2020) Johansson, Kerstin, et al. Evaluation of a new PVC-free catheter material for intermittent catheterization: a prospective, randomized, crossover study. Scandinavian journal of urology 47.1 (2013): 33-37. (Last Accessed September-9-2020)
Johansson, Kerstin, et al. Evaluation of a new PVC-free catheter material for intermittent catheterization: a prospective, randomized, crossover study. Scandinavian journal of urology 47.1 (2013): 33-37. (Last Accessed September-9-2020) Koch, Holger M., et al. Internal exposure of the general population to DEHP and other phthalates—determination of secondary and primary phthalate monoester metabolites in urine. Environmental research 93.2 (2003): 177-185. (Last Accessed September-9-2020)
Koch, Holger M., et al. Internal exposure of the general population to DEHP and other phthalates—determination of secondary and primary phthalate monoester metabolites in urine. Environmental research 93.2 (2003): 177-185. (Last Accessed September-9-2020) Koch, Holger M., et al. Di (2-ethylhexyl) phthalate (DEHP) exposure of voluntary plasma and platelet donors. International journal of hygiene and environmental health 208.6 (2005): 489-498. (Last Accessed September-9-2020)
Koch, Holger M., et al. Di (2-ethylhexyl) phthalate (DEHP) exposure of voluntary plasma and platelet donors. International journal of hygiene and environmental health 208.6 (2005): 489-498. (Last Accessed September-9-2020) Stojanoska, Milica Medic, et al. Do diethyl phthalate (DEP) and di-2-ethylhexyl phthalate (DEHP) influence the metabolic syndrome parameters? Pilot study. Environmental monitoring and assessment 187.8 (2015): 526. (Last Accessed September-9-2020)
Stojanoska, Milica Medic, et al. Do diethyl phthalate (DEP) and di-2-ethylhexyl phthalate (DEHP) influence the metabolic syndrome parameters? Pilot study. Environmental monitoring and assessment 187.8 (2015): 526. (Last Accessed September-9-2020) Gray Jr, L. Earl, et al. Perinatal exposure to the phthalates DEHP, BBP, and DINP, but not DEP, DMP, or DOTP, alters sexual differentiation of the male rat. Toxicological Sciences 58.2 (2000): 350-365. (Last Accessed September-9-2020)
Gray Jr, L. Earl, et al. Perinatal exposure to the phthalates DEHP, BBP, and DINP, but not DEP, DMP, or DOTP, alters sexual differentiation of the male rat. Toxicological Sciences 58.2 (2000): 350-365. (Last Accessed September-9-2020) Stripple, Håkan, Robert Westman, and Daniel Holm. Development and environmental improvements of plastics for hydrophilic catheters in medical care: an environmental evaluation. Journal of Cleaner Production 16.16 (2008): 1764-1776. (Last Accessed September-9-2020)
Stripple, Håkan, Robert Westman, and Daniel Holm. Development and environmental improvements of plastics for hydrophilic catheters in medical care: an environmental evaluation. Journal of Cleaner Production 16.16 (2008): 1764-1776. (Last Accessed September-9-2020) Phthalates Factsheet. National Biomonitoring Program. Centers for Disease Control. (Last Accessed September-9-2020)
Phthalates Factsheet. National Biomonitoring Program. Centers for Disease Control. (Last Accessed September-9-2020)
